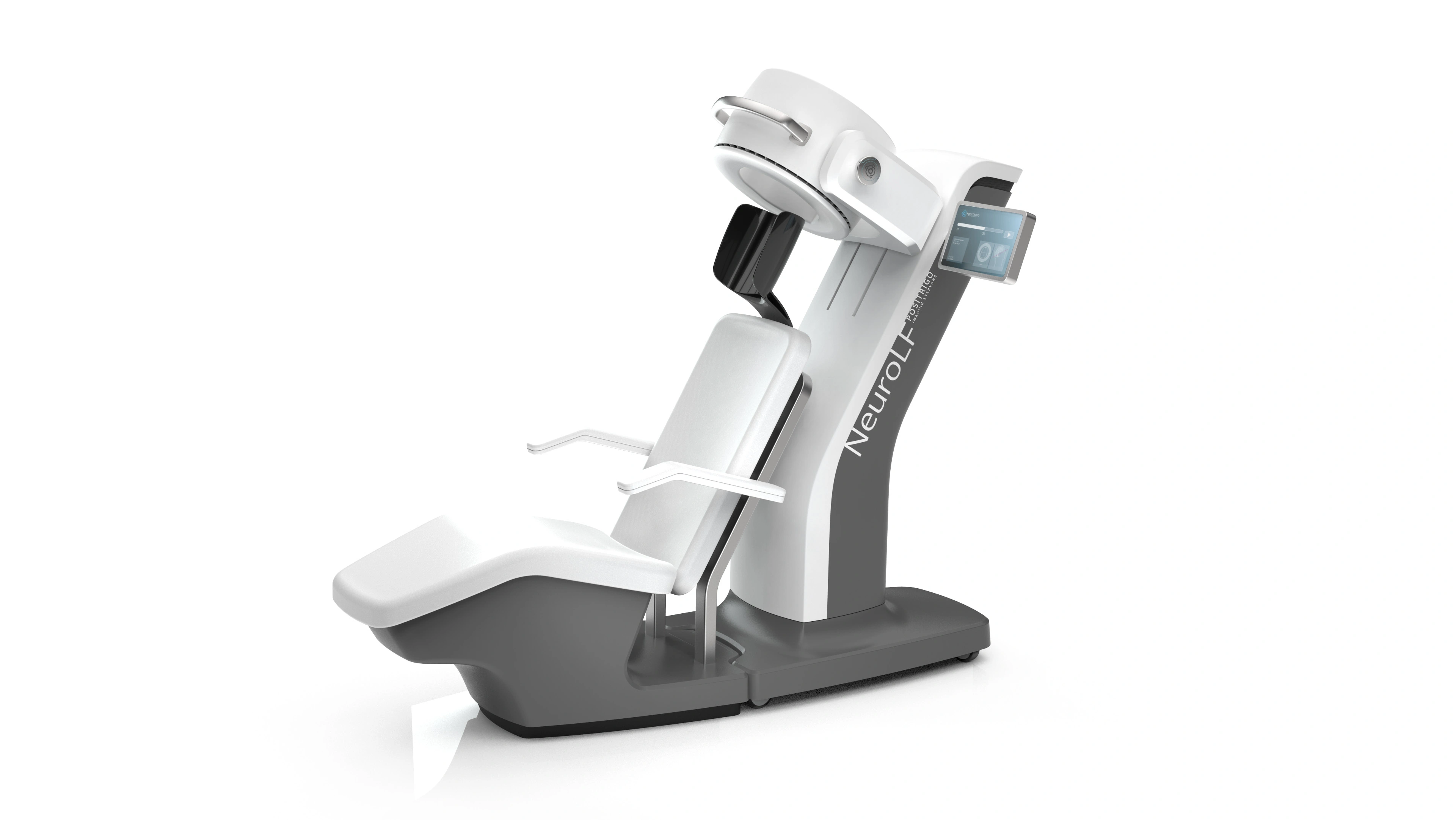
Einfach, intuitiv, ansprechend – wir schaffen eine User Experience, die die Kundschaft und Nutzenden gleichermassen begeistert. Wir gestalten komplexe Anwendungen und Systeme durch intelligente Interaktionsmöglichkeiten und ein ästhetisches Design entsprechend den individuellen Nutzer-Bedürfnissen. Dabei kann es sich um rein digitale Benutzerschnittstellen handeln (VR, Screens) oder auch um Kombinationen von digitalen und physischen Bedienelementen.
Warum UX-Design?
Erfolgreiche Produkte sind selbsterklärend und in der Bedienung intuitiv. Menschen verwenden sie mit Begeisterung und übertragen die positiven Erfahrungen und Erlebnisse direkt auf Ihr Unternehmen.
Diese Produkte entwickeln wir in enger Zusammenarbeit mit unseren Kunden und setzen dabei erfolgreiche und erprobte Methoden ein.
Wir bei Helbling stellen uns täglich komplexen Problemstellungen, um massgeschneiderte Lösungen zu entwickeln. Ein attraktives Design mit Wiedererkennungswert weckt positive Emotionen. Dadurch kann sich der Benutzer mit dem Produkt identifizieren und sich für dieses begeistern!
Wettbewerbsvorteile für unsere Kunden:
- schnelle Orientierung
- hohe Wiedererkennung aller Unternehmenssysteme
- intuitive Bedienung
- konsistente Benutzerführung
- optimierte Bedienabläufe
- kürzere Trainingszeiten
- höhere Produkt-Identifikation
- Kostenreduktion bei der Entwicklung